Simulation and analysis with PipeMaster
This is an R tutorial showing how to simulate data, test models, and estimate parameters using PipeMaster, abc and caret r-packages. The dataset in this tutorial is the same as in Gehara et al in review, and represents 2,177 UCE loci for the neotropical frog Dermatonotus muelleri. We will start working with a subset of the data (200 loci) in the first and second sections of this tutorial, and then use the entire dataset in the third part. For more information about Dermatonotus muelleri see Gehara et al. in review and Oliveira et al. 2018
Overview
- Installation
- First Part: building a model, calculating sumstats and simulating data
- Second Part: visualization and plotting functions
- Third Part: data analysis, approximate Bayesian computation (ABC) & supervised machine-learning (SML)
Installation
PipeMaster can be installed from github using devtools (to get the latest version with the most recent updates), or you can download and install the latest release from my github repository.
-
Installation with devtools Go to the R console, install devtools and then PipeMaster:
install.packages("devtools"") devtools::install_github("gehara/PipeMaster") ## install POPdemog to be able to plot your models install.packages("https://github.com/YingZhou001/POPdemog/raw/master/POPdemog_1.0.3.tar.gz", repos=NULL) -
Installation without devtools Install all dependencies, install PipeMaster latest release from my github. You can do all of this inside the R console using the code below. You may need to check for the latest version and change it in the appropriate line
. install.packages(c("ape","abc","e1071","phyclust","PopGenome","msm","ggplot2","foreach"), repos="http://cran.us.r-project.org") install.packages("http://github.com/gehara/PipeMaster/archive/PipeMaster-0.2.1.tar.gz", repos=NULL) ## install POPdemog to be able to plot your models install.packages("https://github.com/YingZhou001/POPdemog/raw/master/POPdemog_1.0.3.tar.gz", repos=NULL)
CompPhylo workshop starts here!
-
Activate the ssh tunnel
ssh -N -f -L <port>:<node>:<port> <username>@abel.uio.no -
Open your Jupyter notebook following these guidelines. In your Jupyter Notebook tab, go to New on the upper right corner and open a new terminal. In the terminal tab get into R territory:
bash-4.1$ R ##inside R load the pipemaster package library(PipeMaster)
First Part: building a model, calculating sumstats and simulating data
We will download some real data to use as an example. We are going to walk through the basics of the Model Builder (main.menu function) and set up a couple of models. We will run some simulations and calculate summary statistics for the downloaded data. PipeMaster cannot simulate missing data or gaps, so sites with “?”, “N” or “-“ are not allowed. Each locus can have different number of individuals per population as long as there are more than 4.
-
Create a new directory to save the examples
# get the working directory getwd() # create a new directory to save outputs dir.create(paste(getwd(),"/PM_example",sep="")) # set this new folder as your working directory setwd(paste(getwd(),"/PM_example",sep="")) -
Download and unzip the data. The system function allows you to control the OS terminal in order to use bash inside R.
system("wget https://www.dropbox.com/s/y6k9lthazmhea1k/fastas.tar.gz?dl=0") system("mv fastas.tar.gz?dl=0 fastas.tar.gz") system("tar -zxvf fastas.tar.gz") -
Run the code below to see how many files are present in the folder
length(list.files("./fastas")) -
Set up a model by going into the Model Builder. You will be prompted to an interactive menu. Point this function to an object so your model is saved at the end. We are going to set up a 2 population model.
Is <- main.menu() > write bifurcating topology in newick format or 1 for single population:
Main Menu
We start by writing a 2 pop newick: (1,2). This will sep up a 2 pop isolation model with constant population size, no migration. You can follow the description in the menu to add parameters and priors to the model. The numbers on the right indicate the parameters of the model. This model has 2 population size parameters and 1 divergence parameter, or 1 junction in the coalescent direction. We are going to stick with a 3 parameter model for now.
A > Number of populations to simulate 2
B > Tree (go here to remove nodes) (1,2)
Number of nodes (junctions) 1
C > Migration FALSE
D > Pop size change through time FALSE
E > Setup Ne priors
Population size parameters (total) 2
current Ne parameters 2
ancestral Ne parameters 0
F > Setup Migration priors
current migration 0
ancestral migration parameters 0
G > Setup time priors
time of join parameters 1
time of Ne change parameters 0
time of Migration change parameters 0
H > Conditions
I > Gene setup
P > Plot Model
Q > Quit, my model is Ready!
Model Builder>>>>
Ne Priors Menu
Let’s check the Ne priors by typing E in the main menu. In this menu you can see the parameter names and their distribution. PipeMaster has as defaut uniform distributions with min and max values of 100,000 and 500,000 individuals respectively. The name Ne0.pop1 indicates that the parameter is contemporary, hence Ne0. Ancestral parameters will have acending numbers going to the past. For instance, Ne1.pop1 is the ancestral Ne after Ne0, Ne2 is the ancestral Ne after Ne1 and so on. Let’s change one of the priors. Type C and then follow the instructions of the menu.
N > Ne prior distribution: uniform
D > Different ancestral Ne? FALSE
C > current Ne priors min, max
1 Ne0.pop1 1e+05 5e+05
2 Ne0.pop2 1e+05 5e+05
B > Back to main menu
>>>>C
You need to specify the number of the parameter you want to change and then the two parameters of the distribution. Since we are using uniform distribution we need to set up the min and max values. We will change the values for the Ne0.pop1 to min = 2e5, max = 1e6. Then type B to go back to the main.menu
>>>C
Which parameter do you want to set up? (write the reference number from the menu): 1
Ne prior (4Nm) Ne0.pop1 min: 2e5
Ne prior (4Nm) Ne0.pop1 max: 1e6
N > Ne prior distribution: uniform
D > Different ancestral Ne? FALSE
C > current Ne priors min, max
1 Ne0.pop1 2e5 1e6
2 Ne0.pop2 1e+05 5e+05
B > Back to main menu
>>>>B
Time Priors Menu
Type G in the main menu to go to time priors. In the time prior menu you can see all parameters relative to time. In this model we have a single parameter, join1_2, which represents the junction (or divergence in real life direction) of population 1 and 2. The default of PipeMaster is a uniform distribution with min and max of 500,000 and 1,500,000 generations. Time is measured in generations. If your organism has a generation time different than 1, you will need to convert the time from years to generations in order to set up your prior. For example, if you want to setup a divergence between 100,000 and 1,000,000 years and your organism has a generation time of 4 years, you will need to divide the time by 4. Your min and max values will be 25,000 ans 250,000. Type B to go back to the main menu.
P > Time prior distribution: uniform
Time priors Min - Max
J > time of junctions:
1 join1_2 5e+05 1500000
B > Back to Ne menu
Conditions Menu
Type H in the main menu to go to the conditions menu. Here you get a list of parameters and some options. You can use the options represented by the letters to set up a condition on your parameters. For example if you want Ne0.pop1 to be always larger than Ne0.pop2 and their priors overlap you can go to option S and setup a size condition. Let’s try this.
Model Builder >>>> H
size parameter -- Ne0.pop1
size parameter -- Ne0.pop2
time parameter -- join1_2
S > place a size condition
T > place a time condition
1 > see size matrix
3 > see time matrix
B > back to main menu
>>>>S
Matrix of parameter conditions
This matrix indicates the parameter conditions where NA means there is no condition. Let’s try to set up a condition. We want Ne0.pop1 to be always larger than Ne0.pop2, so we need to write Ne0.pop1 > Ne0.pop2 as explaned in the menu.
Ne0.pop1 Ne0.pop2
Ne0.pop1 0 NA
Ne0.pop2 NA 0
Write the name of 2 parameters with a logic sign inbetween ( > or < or = ) separated by a space.
Ex.: Ne0.pop1 < Ne0.pop2
Ne0.pop1 > Ne0.pop2
size parameter -- Ne0.pop1
size parameter -- Ne0.pop2
time parameter -- join1_2
S > place a size condition
T > place a time condition
1 > see size matrix
3 > see time matrix
B > back to main menu
>>>>
Now you can see the size matrix by typing 1 in the menu. You can see that the matrix indicates that Ne0.pop1 > Ne0.pop2.
>>>>1
Ne0.pop1 Ne0.pop2
Ne0.pop1 "0" ">"
Ne0.pop2 "<" "0"
[1] "-----------------"
size parameter -- Ne0.pop1
size parameter -- Ne0.pop2
time parameter -- join1_2
S > place a size condition
T > place a time condition
1 > see size matrix
3 > see time matrix
B > back to main menu
>>>>
Gene Menu
Type I in the main menu to go to the gene menu. To get into the gene menu you will need to answer two questions. What type of data you want to simulate (sanger or genomic)? and how many loci?. We will simulate 200 UCE loci according to the downloaded data. Thus we answer genomic or g and 200.
Model Builder >>>>I
What type of data to simulate (sanger or genomic)?:genomic
how many loci to simulate? You should include all invariable locus: 200
M > Mutation rate prior distribution: uniform
P > priors Min - Max
1e-11 1e-09
1 > number of loci
200
B > Back to main menu
>>>>
Mutation rate prior
In the case of genomic data the mutation rate works as a hyperparameter. The defaut uniform distribution above indicates the min and max values to sample an average and SD of all mutation rates. That is, the actual mutation rate for each of the 200 loci will be sampled from a normal distribution with average and SD sampled from this uniform prior. In each simulation iteration a new average and SD are sampled and from these parameters the 200 mutation rates are sampled. This normal distribution is truncated at zero, so it doesn’t not really always have a bell shape. You can set different distribution for the mutation rate. All distributions available in R are allowed, but this distribution is specified in the simulation function (sim.msABC.sumstat). We will see this further in the tutorial. Now go back to the main menu and hit Q to get out of the Model Builder.
Generating a model from a template
Our model was saved in the object Is. We can easily generate another model using a previous model as template. We are going to use our Is model to generate a similar model but with gene flow. Let’s call this model IM, isolation with migration. We will go directly to the main menu. We will then type C to include migration parameters, than type y or yes.
IM <- main.menu(Is)
A > Number of populations to simulate 2
B > Tree (go here to remove nodes) (1,2)
Number of nodes (junctions) 1
C > Migration FALSE
D > Pop size change through time FALSE
E > Setup Ne priors
Population size parameters (total) 2
current Ne parameters 2
ancestral Ne parameters 0
F > Setup Migration priors
current migration 0
ancestral migration parameters 0
G > Setup time priors
time of join parameters 1
time of Ne change parameters 0
time of Migration change parameters 0
H > Conditions
I > Gene setup
P > Plot Model
Q > Quit, my model is Ready!
Model Builder >>>>C
----------------------------------------------
Migration among populations (YES or NO)?: Y
A > Number of populations to simulate 2
B > Tree (go here to remove nodes) (1,2)
Number of nodes (junctions) 1
C > Migration TRUE
D > Pop size change through time FALSE
E > Setup Ne priors
Population size parameters (total) 2
current Ne parameters 2
ancestral Ne parameters 0
F > Setup Migration priors
current migration 2
ancestral migration parameters 0
G > Setup time priors
time of join parameters 1
time of Ne change parameters 0
time of Migration change parameters 0
H > Conditions
I > Gene setup
P > Plot Model
Q > Quit, my model is Ready!
Model Builder >>>>
Migration prior menu
In option we can go into the migration menu. Migration is measured in 4Nmij where mij is the fraction of individuals in population i made up of migrants from population j.
M > Migration prior distribution: uniform
D > Different ancestral migration? FALSE
P > priors Min - Max
1 mig0.1_2 0.1 1
2 mig0.2_1 0.1 1
B > Back to main menu
>>>>
Let’s go back to the main menu and then quit. The model is saved in the object IM.
Model Object
By typing the model name in the R console we can see its contents. You don’t have to know how to read the model object, but it might help you understand how the package works. The loci index shows your loci with mutation rates. the I index shows the population structure, the third column indicates the number of pops and the following columns indicate the number of individuals for each population. The flags index shows the parameters of your model with respective priors. The conds index shows the condition matrices and the tree parameter shows the topology of the model.
> Is
$loci
[,1] [,2] [,3] [,4] [,5] [,6]
[1,] "genomic" "bp" "200" "1e-11" "1e-09" "runif"
$I
[,1] [,2] [,3] [,4] [,5]
[1,] "genomic" "-I" "2" NA NA
$flags
$flags$n
[,1] [,2] [,3] [,4] [,5] [,6]
[1,] "Ne0.pop1" "-n" "1" "1e+05" "5e+05" "runif"
[2,] "Ne0.pop2" "-n" "2" "1e+05" "5e+05" "runif"
$flags$ej
[,1] [,2] [,3] [,4] [,5] [,6]
[1,] "join1_2" "-ej" "1 2" "5e+05" "1500000" "runif"
$conds
$conds$size.matrix
Ne0.pop1 Ne0.pop2
Ne0.pop1 "0" ">"
Ne0.pop2 "<" "0"
$conds$time.matrix
join1_2
join1_2 0
$tree
[1] "(1,2)"
attr(,"class")
[1] "Model"
Checking model parameters and manipulating prior values
There is an easier way to check the model parameters and priors. You can also update your prior without using the main.menu function. To see your priors use get.prior.table. This function generates a table with the model parameters and priors. You can then alter this table and use it to update the prior values of your model using update.priors. Note that for the update.prior to work, the parameters in the table and model object have to be the same.
> Is.tab <- get.prior.table(model=Is)
> Is.tab
Parameter prior.1 prior.2 distribution
1 Ne0.pop1 1e+05 5e+05 runif
2 Ne0.pop2 1e+05 5e+05 runif
3 join1_2 5e+05 1500000 runif
> Is.tab[,2:3] <- 1000
> Is.tab
Parameter prior.1 prior.2 distribution
1 Ne0.pop1 1000 1000 runif
2 Ne0.pop2 1000 1000 runif
3 join1 1000 1000 runif
> Is2 <- PipeMaster:::update.priors(tab = Is.tab, model = Is)
> get.prior.table(model=Is2)
Parameter prior.1 prior.2 distribution
1 Ne0.pop1 1000 1000 runif
2 Ne0.pop2 1000 1000 runif
3 join1 1000 1000 runif
Saving and reloading a model
You can save the model as a text file using dput. To read the model back to R use dget.
dput(Is, "Is.txt")
Is <- dget("Is.txt")
Replicating the empirical data structure to the model
To have the model ready for the simulation we need to replicate the data structure to the model. We need to setup the exact number of individuals per population and the length of each locus. To do this we use the get.data.structure function. This function needs: (i) an assignment file, a two column data frame with the name of the individuals and their respective population; and (ii) a model object where the structure will be replicated. We will download an assignment file from my dropbox.
system("wget https://www.dropbox.com/s/x46xjd85yznijdb/assignments.txt?dl=0")
system("mv assignments.txt?dl=0 assignments.txt")
popassign <- read.table("assignments.txt", header=T)
Is <- get.data.structure(model = Is, path.to.fasta = "./fastas", pop.assign = popassign, sanger = F)
IM <- get.data.structure(model = IM, path.to.fasta = "./fastas", pop.assign = popassign, sanger = F)
## !!! save the models!! ##
dput(Is, "Is.txt")
dput(IM, "IM.txt")
Summary statistics calculation
To calculate the summary statistics for the empirical data we will use the obs.sumstat.ngs function. This function also needs an assignment file and a model object. By default PipeMaster calculates all the available summary statistics, you select them a posteriori.
obs <- obs.sumstat.ngs(model = Is, path.to.fasta = "./fastas", pop.assign = popassign)
To see the observed summary stats just type obs. To see the name of the summary stats colnames(obs)
> colnames(obs)
[1] "s_average_segs_1" "s_variance_segs_1"
[3] "s_average_segs_2" "s_variance_segs_2"
[5] "s_average_segs" "s_variance_segs"
[7] "s_average_pi_1" "s_variance_pi_1"
[9] "s_average_pi_2" "s_variance_pi_2"
[11] "s_average_pi" "s_variance_pi"
[13] "s_average_w_1" "s_variance_w_1"
[15] "s_average_w_2" "s_variance_w_2"
[17] "s_average_w" "s_variance_w"
[19] "s_average_tajd_1" "s_variance_tajd_1"
[21] "s_average_tajd_2" "s_variance_tajd_2"
[23] "s_average_tajd" "s_variance_tajd"
[25] "s_average_ZnS_1" "s_variance_ZnS_1"
[27] "s_average_ZnS_2" "s_variance_ZnS_2"
[29] "s_average_ZnS" "s_variance_ZnS"
[31] "s_average_Fst" "s_variance_Fst"
[33] "s_average_shared_1_2" "s_variance_shared_1_2"
[35] "s_average_private_1_2" "s_variance_private_1_2"
[37] "s_average_fixed_dif_1_2" "s_variance_fixed_dif_1_2"
[39] "s_average_pairwise_fst_1_2" "s_variance_pairwise_fst_1_2"
[41] "s_average_fwh_1" "s_variance_fwh_1"
[43] "s_average_fwh_2" "s_variance_fwh_2"
[45] "s_average_FayWuH" "s_variance_FayWuH"
[47] "s_average_dvk_1" "s_variance_dvk_1"
[49] "s_average_dvh_1" "s_variance_dvh_1"
[51] "s_average_dvk_2" "s_variance_dvk_2"
[53] "s_average_dvh_2" "s_variance_dvh_2"
[55] "s_average_dvk" "s_variance_dvk"
[57] "s_average_dvh" "s_variance_dvh"
[59] "s_average_thomson_est_1" "s_variance_thomson_est_1"
[61] "s_average_thomson_est_2" "s_variance_thomson_est_2"
[63] "s_average_thomson_est" "s_variance_thomson_est"
[65] "s_average_thomson_var_1" "s_variance_thomson_var_1"
[67] "s_average_thomson_var_2" "s_variance_thomson_var_2"
[69] "s_average_thomson_var" "s_variance_thomson_var"
[71] "s_average_pi_1_s_average_w_1" "s_variance_pi_1_s_variance_w_1"
[73] "s_average_pi_2_s_average_w_2" "s_variance_pi_2_s_variance_w_2"
[75] "s_average_pi_s_average_w" "s_variance_pi_s_variance_w"
There are 76 summary statistics for this data, these are averages and variances across loci, for each population and overall. We are going to use grep to select the stats we want to exclude. Descriptions of summary statistics are found in the msABC manual
cols <- c(grep("thomson", colnames(obs)),
grep("pairwise_fst", colnames(obs)),
grep("Fay", colnames(obs)),
grep("fwh", colnames(obs)),
grep("_dv", colnames(obs)),
grep("_s_", colnames(obs)),
grep("_ZnS", colnames(obs)))
obs <- t(data.frame(obs[,-cols]))
Check the sumstats names again
> colnames(obs)
[1] "s_average_segs_1" "s_variance_segs_1"
[3] "s_average_segs_2" "s_variance_segs_2"
[5] "s_average_segs" "s_variance_segs"
[7] "s_average_pi_1" "s_variance_pi_1"
[9] "s_average_pi_2" "s_variance_pi_2"
[11] "s_average_pi" "s_variance_pi"
[13] "s_average_w_1" "s_variance_w_1"
[15] "s_average_w_2" "s_variance_w_2"
[17] "s_average_w" "s_variance_w"
[19] "s_average_tajd_1" "s_variance_tajd_1"
[21] "s_average_tajd_2" "s_variance_tajd_2"
[23] "s_average_tajd" "s_variance_tajd"
[25] "s_average_ZnS" "s_variance_ZnS"
[27] "s_average_Fst" "s_variance_Fst"
[29] "s_average_shared_1_2" "s_variance_shared_1_2"
[31] "s_average_private_1_2" "s_variance_private_1_2"
[33] "s_average_fixed_dif_1_2" "s_variance_fixed_dif_1_2"
Save the observed as a table using write.table.
write.table(obs,"observed.txt", quote=F,col.names=T, row.names=F)
Simulating Data
Now that we have two models, Is and IM we are going to simulate summary statistics. We will use the sim.msABC.sumstat to simulate genomic data. This function only works in linux and Mac. PipeMaster controls msABC to simulate the data. It simulates data in batches or blocks to avoid memory overload in R and at the same time optimize the time spent in writing the simulations to file. To control the total number of simulations you have to control the size of the simulation block, the number of blocks to simulate, and the number of cores used. The total number of simulations = nsim.blocks x block.size x ncores. You can play with these values to optimize the speed of the simulation process. A small block size will take less RAM but will require a more frequent management of the slave nodes by the master node. This can be time consuming. A large block size may overload R, R can’t handle a lot of memory very well. It can also take up too much RAM, specially if you are running several cores at the same time. PipeMaster will output a time estimate at the console. This might help you optimize the parameters. From my experience, a block.size of 1000 will be good for most cases. If you don’t want to mess with this, just leave at 1000, it should work fine.
sim.msABC.sumstat(Is, nsim.blocks = 1, use.alpha = F, output.name = "Is", append.sims = F,
block.size = 500, ncores = 2)
sim.msABC.sumstat(IM, nsim.blocks = 1, use.alpha = F, output.name = "IM", append.sims = F,
block.size = 500, ncores = 2)
Second part: visualizations and plotting functions
In this part of the tutorial we will go through some of the visualization functions of PipeMaster. We are going to use the notebook tab of Jupyter notebook to visualize the plots in an interactive way.
-
Open a new notebook by going to New in the upper right corner of the main Jupyter Notebook tab.
-
To run R in jupyter notebook we need to load rpy2
%load_ext rpy2.ipython -
From now on every chunk of R code you want to run should start with %%R.
- Load PipeMaster, check the working directory and list the files.
%%R suppressMessages(library(PipeMaster)) suppressMessages(library(POPdemog)) list.files() - change working directory to PM_example, if needed, and list files
%%R setwd("./PM_example") list.files()
Plotting a Model
There is now a new function in PipeMaster to plot your model. This function is a wrapper of the PlotMS function from the POPdemog r-package. I have not tested it extensively yet, if you find bugs please send me an email (marcelo.gehara@gmail.com).
%%R
Is <- dget("Is.txt")
IM <- dget("IM.txt")
PlotModel(model=Is, use.alpha = F, average.of.priors=F)
PlotModel(model=Is, use.alpha = F, average.of.priors=T)
PlotModel(model=IM, use.alpha = F, average.of.priors=F)
PlotModel(model=IM, use.alpha = F, average.of.priors=T)
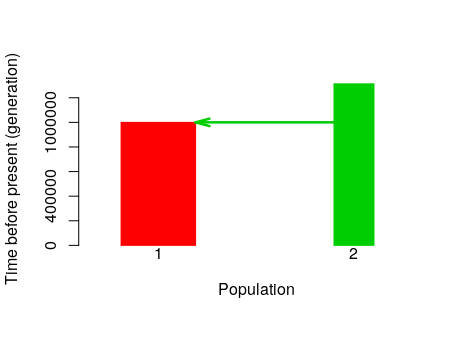
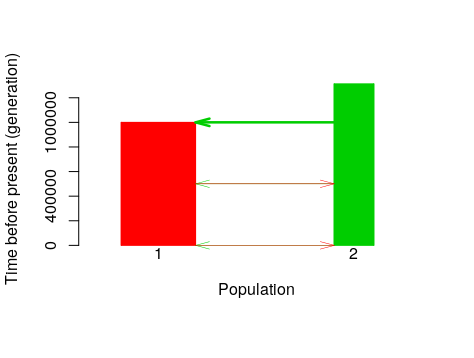
Visualize prior distributions
We can use the plot.prior function to visualize the prior distributions.
%%R
PipeMaster:::plot.priors(Is, nsamples = 10000)
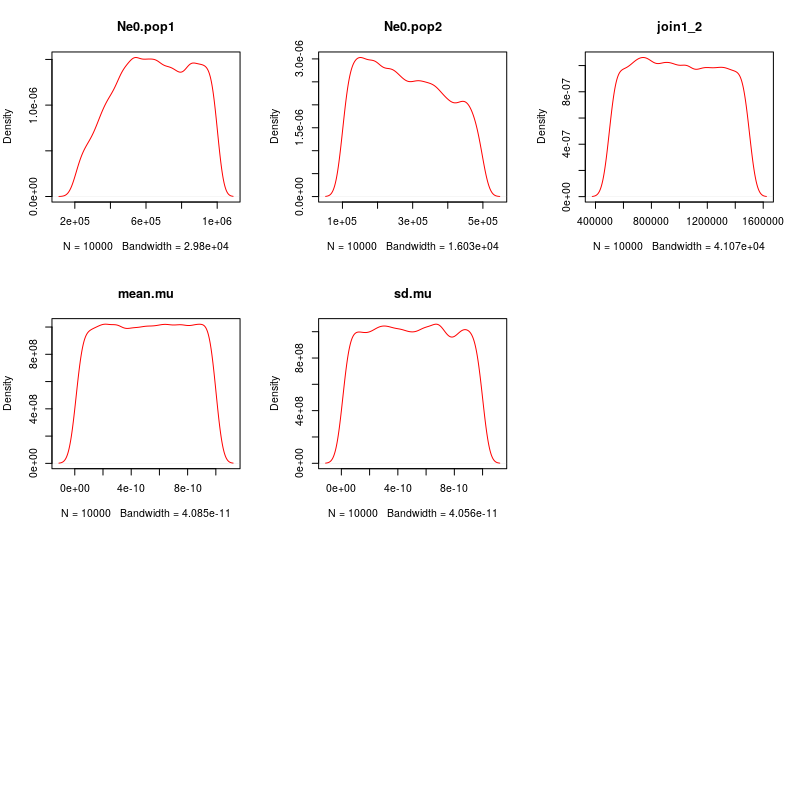
Plotting simulations against empirical data
Let’s visualize the simulations. Read the simulations back into R. If your simulation file is very big (you have many simulations, like 5E5 or more) you should use the bigmemory r-package to handle the data. We will also match the simulations sumstats to the observed so that we keep the same set of sumstats in the simulated.
%%R
Is.sim <- read.table("SIMS_Is.txt", header=T)
IM.sim <- read.table("SIMS_IM.txt", header=T)
obs <- read.table("observed.txt", header=T)
Is.sim <- Is.sim[,colnames(Is.sim) %in% colnames(obs)]
IM.sim <- IM.sim[,colnames(IM.sim) %in% colnames(obs)]
Now we can plot the observed againt the simulated. This helps you evaluate your model and have a visual idea of how the simulations fit the empirical data.
%%R
PipeMaster:::plot.sim.obs(Is.sim, obs)
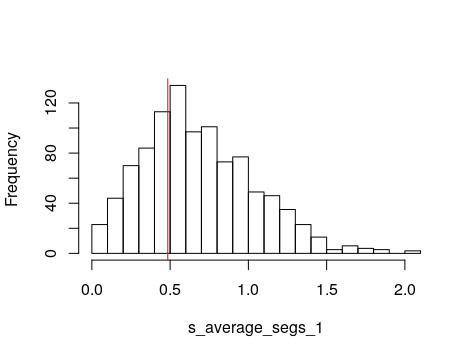
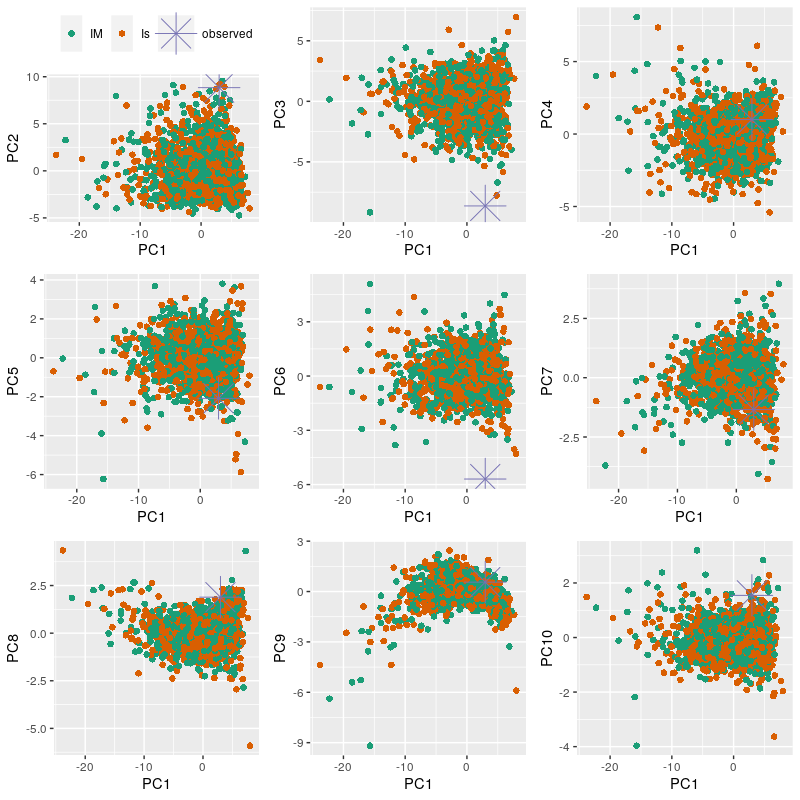
Plotting a PCA
We can also plot a Principal Component Analysis of the simulations against the empirical data. This also helps evaluating the fit of your models. First we will combine the models in a single data frame and we will generate an index.
%%R
models <- rbind(Is.sim, IM.sim)
index <- c(rep("Is", nrow(Is.sim)), rep("IM", nrow(IM.sim)))
plotPCs(model = models, index = index, observed = obs, subsample = 1)
 —————————————————————————————————–
—————————————————————————————————–
Third part: approximate Bayesian computation (ABC) & supervised machine-learning (SML)
In the last part of the tutorial we are going to perform the data analysis using PipeMaster, abc and caret r-packages.
-
This part of the tutorial is limited to a single case, you should latter check the following materials if you plan to perform any of these analyses:
-
(i) The vignette of the abc is very informative and covers the entire package.
-
(ii) caret is a very extensive package for machine-learning, there are hundreds of algorithms avalable with extensive online documentation.
-
(iii) Here is a series of YouTube videos explaning Neural Networks in much more detail, I took some of the material from these videos.
abc is already a dependency of PipeMaster but we need to load caret. To run caret in parallel we need to load a r-package that manages nodes to run loops in parallel using MPI. There are several r-packages for this, we are going to use doMC.
%%R
library(caret) # caret: used to perform the superevised machine-learning (SML)
library(doMC) # doMC: necessary to run the SML in parallel
Load the example data available in PipeMaster. This example data is based on Gehara et al. in review which is the paper that describes the package. Here you can access a spread sheet with all model parameters and priors.
%%R
# observed summary statistics
data("observed_Dermatonotus", package = "PipeMaster")
# models used in Gehara et al
data("models", package="PipeMaster")
There are 10 models. Let’s plot one of these models. Remmeber, you can use them as templates for your own analysis. You will just need to update the priors as above, and replicate your data structure to the model using the get.data.structue as explaned above. To see all the model objects type ls() in the R console.
%%R
PlotModel(model=IsBot2, use.alpha=c(T,1), average.of.priors=T)
Simulate data for 4 of the 10 models. We are not going to simulate all models since it would take too much time. I simulated all of these models for the frog Dermatonotus muelleri in the paper that describes the package.
%%R
sim.msABC.sumstat(Is, nsim.blocks = 2, use.alpha = F, output.name = "Is", append.sims = F,
block.size = 100, ncores = 10)
sim.msABC.sumstat(IM, nsim.blocks = 2, use.alpha = F, output.name = "IM", append.sims = F, block.size = 100, ncores = 10)
sim.msABC.sumstat(IsBot2, nsim.blocks = 2, use.alpha = c(T,1), output.name = "IsBot2",
append.sims = F, block.size = 100, ncores = 10)
sim.msABC.sumstat(IMBot2, nsim.blocks = 2, use.alpha = c(T,1), output.name = "IMBot2",
append.sims = F, block.size = 100, ncores = 10)
Now that the simulations are done we can read them back into R and select the summary statistics we are going to use.
%%R
Is.sim <- read.table("SIMS_Is.txt", header=T)
IM.sim <- read.table("SIMS_IM.txt", header=T)
IsBot2.sim <- read.table("SIMS_IsBot2.txt", header=T)
IMBot2.sim <- read.table("SIMS_IMBot2.txt", header=T)
Select sumstats
%%R
cols <- c(grep("thomson", names(observed)),
grep("pairwise_fst", names(observed)),
grep("Fay", names(observed)),
grep("fwh", names(observed)),
grep("_dv", names(observed)),
grep("_s_", names(observed)),
grep("_ZnS", names(observed)))
observed <- observed[-cols]
colnames(observed)
[1] "s_average_segs_1" "s_variance_segs_1" "s_average_segs_2"
[4] "s_variance_segs_2" "s_average_segs" "s_variance_segs"
[7] "s_average_pi_1" "s_variance_pi_1" "s_average_pi_2"
[10] "s_variance_pi_2" "s_average_pi" "s_variance_pi"
[13] "s_average_w_1" "s_variance_w_1" "s_average_w_2"
[16] "s_variance_w_2" "s_average_w" "s_variance_w"
[19] "s_average_tajd_1" "s_variance_tajd_1" "s_average_tajd_2"
[22] "s_variance_tajd_2" "s_average_tajd" "s_variance_tajd"
[25] "s_average_ZnS" "s_variance_ZnS" "s_average_Fst"
[28] "s_variance_Fst" "s_average_shared_1_2" "s_variance_shared_1_2"
[31] "s_average_private_1_2" "s_variance_private_1_2" "s_average_fixed_dif_1_2"
[34] "s_variance_fixed_dif_1_2"
Combine simulations in a single matrix matching the summary stats names in the observed and run a Principal Components Analyses to visualize model-fit
%%R
models <- rbind(Is.sim[,colnames(Is.sim) %in% names(observed)],
IM.sim[,colnames(IM.sim) %in% names(observed)],
IsBot2.sim[,colnames(IsBot2.sim) %in% names(observed)],
IMBot2.sim[,colnames(IMBot2.sim) %in% names(observed)])
data <- c(rep("Is", nrow(Is.sim)),
rep("IM", nrow(IM.sim)),
rep("IsBot2", nrow(IsBot2.sim)),
rep("IMBot2", nrow(IMBot2.sim)))
plotPCs(model = models, index = data, observed = observed, subsample = 0.5)
Supervised machine-learning analysis for model classification.
We are going to train a neural network algorithm and then use it to classify our empirical data. In the paper that describes the package, Gehara et al. in review, I ran a simulation experiment to compare ABC rejection with SML with neural network and I found that the SML is much more efficient and accurate.
To train the algorithm we need to split the data into training and testing, usually 75% is used for training and 25% for testing. We can do that with createDataPartition. We also need to specify our predictors and our outcome. In our case, the predictors are the summary statistics and the outcome is the model label or index.
It can be very time consuming to set up the parameters of the neural network, and there is no objective way to decide which parameters values to use. To help deciding the parameter values, caret runs the training multiple times with different parameters values to see which combination gets the highest accuracy in model selection. And it does this using different resampling methods. You can set up the number of replicates and the resampling method used in the trainControl function.
%%R
# set up number of cores for SML
registerDoMC(1)
## combine simulations and index
models <- cbind(models,data)
## setup the outcome (name of the models, cathegories)
outcomeName <- 'data'
## set up predictors (summary statistics)
predictorsNames <- names(models)[names(models) != outcomeName]
## split the data into trainning and testing sets; 75% for trainning, 25% testing
splitIndex <- createDataPartition(models[,outcomeName], p = 0.75, list = FALSE, times = 1)
train <- models[ splitIndex,]
test <- models[-splitIndex,]
## bootstraps and other controls
objControl <- trainControl(method='boot', number=1, returnResamp='final',
classProbs = TRUE)
## train the algoritm
nnetModel_select <- train(train[,predictorsNames], train[,outcomeName],
method="nnet",maxit=5000,
trControl=objControl,
metric = "Accuracy",
preProc = c("center", "scale"))
## predict outcome for testing data, classification
predictions <- predict(object=nnetModel_select, test[,predictorsNames], type='raw')
## calculate accuracy in model classification
accu <- postResample(pred=predictions, obs=as.factor(test[,outcomeName]))
## predict probabilities of the models for the observe data
pred <- predict(object=nnetModel_select, observed, type='prob')
# visualize results
t(c(pred,accu))
# write results to file
write.table(c(pred,accu),"results.selection.txt")
Visualize and write results to file
%%R
# visualize results
t(c(pred,accu))
# write results to file
write.table(c(pred,accu),"results.selection.txt")
Approximate Bayesian computation with neural network for parameter estimate.
The abc performs a rejection step and then uses the retained data to train a neural network to estimate parameters
%%R
# read selected model
IsBot2.sim <- read.table("SIMS_IsBot2.txt", header=T)
# separate summary statistics from parameters
sims <- IsBot2.sim[,colnames(IsBot2.sim) %in% colnames(observed)]
param <- IsBot2.sim[,1:11]
# estimate posterior distribution of parameters
post <- abc(target = observed,
param = param,
sumstat = sims,
sizenet = 20,
method = "neuralnet",
MaxNWts = 5000,
tol=0.1)
# adjust tolerance level according to the total number of simulations.
# write results to file
write.table(summary(post), "parameters_est.txt")
Plot posterior distribution
%%R
# plot posterior probabilities
plot(post, param = param)
Cross-validation
It is important to perform a cross-validation to evaluate if we can estimate the parameters with confidence. The function cv4abc performs a leave-one-out experiment to evaluate the performance of the method. To correctly do this we have to use the same tolerace value and method with the same parameters as used above.
%%R
# cross-validation for parameter estimates
cv <- cv4abc(param = param,
sumstat = sims,
nval = 20,
sizenet = 20,
method = "neuralnet",
MaxNWts = 5000,
tol = 0.1)
plot(cv)
Check the error of the estimate
%%R
summary(cv)
Codemographics
Check the hABC manual if you want to try a codemographic analysis with single-locus data